are homogeneous mixtures always pure substances
A homogeneous mixture is not considered a pure substance because it can be separated through mechanical and thermal processes. A pure substance containing only one kind of atom An element is always uniform all the way through homogeneous.
What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples
All physical objects are made up of chemical substances which are unchanging in chemical composition and characteristics.

. When a homogeneous substance consists of the same type of molecule with fixed and uniform composition throughout then the. Is a compound a homogeneous or heterogeneous. Play this game to review undefined.
A Is the sand an example of a mixture or pure substance. A pure substance however cannot be separated. Pure Substances Are Made Up of One Type of Particle.
Difference Between Pure Substance and Homogeneous Mixture Pure Substance vs Homogeneous Mixture Matter is composed of different substances like atoms and other molecules that have volume and mass. Please do not block ads on this website. Brass is a mixture of Cu and Zn.
Extra Credit Station 11 - Melted Crayons a Are these melted crayons a mixture or pure substance. Homogeneous mixtures have same composition anywhere throughout the mixture. Mixtures can be classified as homogeneous or heterogeneous.
Are homogeneous mixtures always pure substances. Every unit of the homogeneous mixture is likely to be every other unit. Examples of homogeneous mixtures include vegetable oil honey and air.
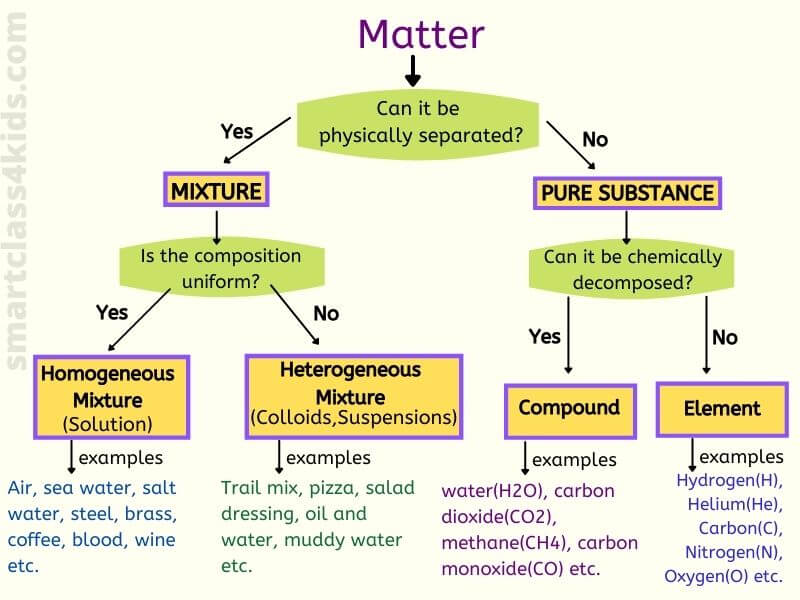
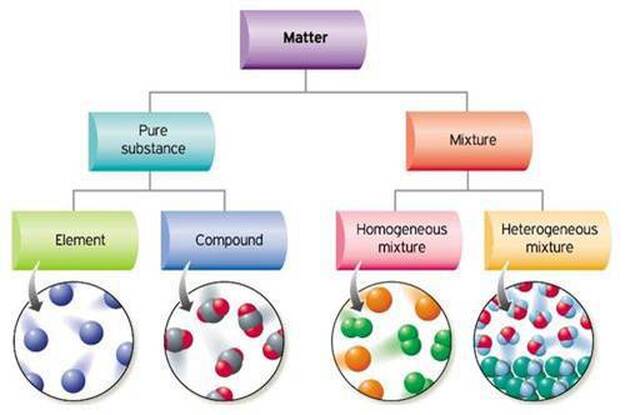
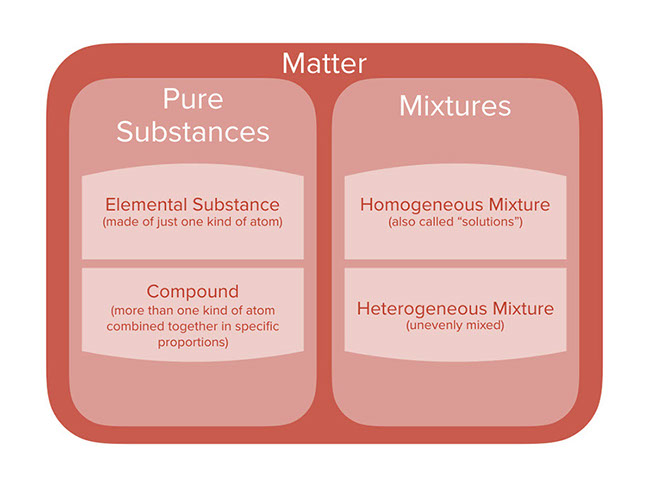
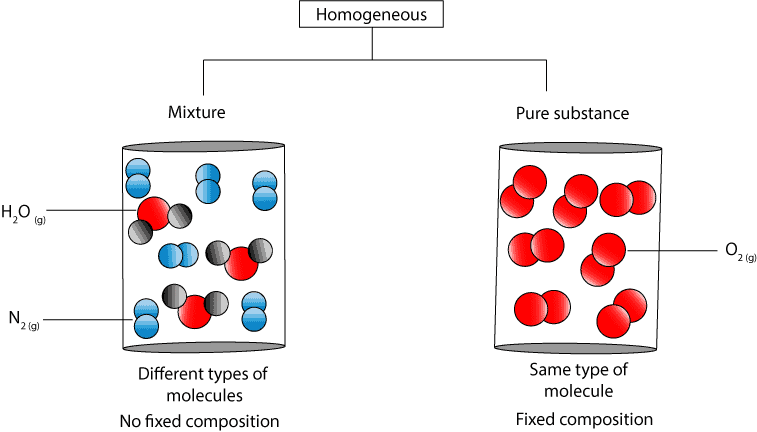
Compounds are homogeneous forms of matter. But on the other hand pure substances are those substances that cannot be separated by physical process without breaking its chemical chain. Mixtures are physical combinations of two or more elements andor compounds.
On the other hand there are homogeneous mixtures. Pure iron is very soft and stretches when hot but with c it forms steel which is hard and strong. Mixtures are composed of variable proportions of molecules and atoms.
Dry ice is solid carbon dioxide or CO2. It is found that homogenous mixtures can be separated easily by using various physical methods. By definition a pure substance or a homogeneous mixture consists of a single phase.
They look pure to a non-science student but actually are mixture of various atoms and molecules having no boundaries of separation between the molecules or atoms that is the constituents. Salt water mixture tastes the same all over the mixture. - As we know that homogenous term indicates that the substance has the same composition throughout.
Also Know are all substances homogeneous. Compounds are pure substances the are composed of two or more elements chemically bonded. Well according to the theory of the father of chemistry which is Jon mcdale says that if u multiply 20 by 2 then - 5 calculate the percentage to the nearest tenth multiply by 100 then it will give.
B Is the sugar water a heterogeneous or homogeneous mixture. Are compounds heterogeneous or homogeneous Why. C What state of matter is Station 10.
When oil and water are combined they do not mix evenly but instead form two separate layers. This is because pure substances are made up of one type of particle. Mixtures can be separated into their components by chemical or physical means.
No matter the amount of a pure substance you have it will always have the same properties. They have definite physical and. Therefore a homogeneous substance can either be a mixture or a pure substance.
Homogeneous Mixtures are the ones that possess the same combination and properties throughout their mass. Pure substances and mixtures. Bronze is a mixture of Cu and TinIt is toughresistant to corrosionused to make statuescoinsmedalsutensils.
Dry ice is a _____. Mixtures always have variable compositions whereas compounds have a fixed and definite composition. A pure substance cant be a mixture.
Heterogeneous substances are always mixtures. Homogeneous means the same or uniform throughout. That fact itself indicates that it is not a pure substance.
A heterogeneous mixture consists of two or more phases. A pure substance is a form of matter that has a fixed chemical composition and a distinct characteristic while a homogeneous mixture is a mixture of two or more compounds with compositions that are uniform or mixed together in such a way that they are indistinguishable from each other. A pure substance is a substance that has definite physical and chemical properties such as appearance melting point and reactivity.
D How can I turn water into a gas. The homogeneous mixture of two or more metals is called alloy. Depending on who you talk to homogeneous mixtures may be considered examples of pure substances.
It just contains water. The pure substances They are those that have a constant chemical composition that is a composition that does not vary even if the physical conditions to which these substances are subjected change. Are elements always homogeneous.
Dry ice may be used as a cooling agent and contains two oxygen atoms bonded to a carbon atom. While these substances contain multiple types of molecules their composition is consistent throughout a sample. A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample.
A substance is homogeneous if its composition is identical wherever you sample it - it has uniform composition and properties throughout. Mixtures are heterogeneous forms of matter. If you add soot to air it ceases to be a pure substance.
All the matter that we know of in the universe can be classified according to its constitution into two categories although there are also other classifications. No ads no money for us no free stuff for. What are the two types of alloys.
A chemical substance is also known as a pure. B Is this an example of a chemic al or physical. Understanding the difference between pure substances and mixtures and knowing how to separate mixtures into their component pure substances is important not only to Chemistry but to maintaining your own health.
First of all it is a mixture. Pure substances are homogeneous Pure substance cannot be separated into simpler substances by physical methods Mixtures have variable composition Mixtures usually have small medium large size particles The property of pure substance does not vary Solid substance has a sharp melting point Solid mixture met at temperature range.
Pure Substances And Mixtures Neds Declassified

Chapter Two Properties Of Matter Matter Pure Substance Elementcompoundmixture Homogeneous Mixture Solution Heterogeneous Mixture Colloidsuspension Classification Ppt Download

What Are The Types Of Pure Substances And Mixtures A Plus Topper

Homogeneous Mixture Examples Chemistry

Pure Substances Mixtures And Solutions Oh My Ppt Download
Unit 3 Pure Substances And Mixtures San Francisco De Paula Science Department

Classification Of Matter Ultimate Card Sort For Pure Substances And Mixtures Sorting Cards Creative Teaching Teacher Preparation

1 3 Classifying Matter According To Its Composition Chemistry Libretexts

Selina Concise Chemistry Class 6 Icse Solutions Chapter 5 Pure Substances And Mixtures Separation Of Mixtures Ncert Chemistry Class Chemistry Science Notes
Lesson Categories Of Chemicals And Mixtures
Pure Substance Vs Mixture Ppt Video Online Download

What Is A Heterogeneous Mixture Definition And Examples Heterogeneous Mixture Food Science Homogeneous Mixture

Compound Vs Mixture Difference And Comparison Diffen Compounds Science Chemistry Classroom Chemical Science
If A Substance Is Homogeneous Is It A Pure Substance
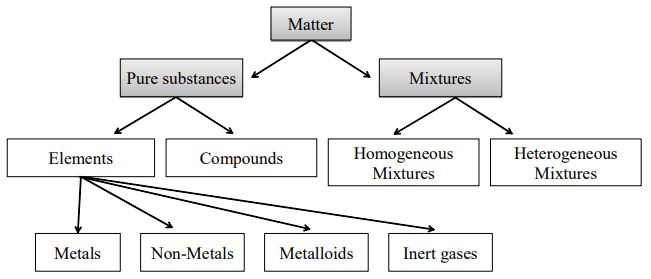
Is Matter Around Us Pure Practically Study Material

Pure Substances And Mixtures Chemistry For Kids Mocomi Chemistry For Kids Pure Products Learning Science

Mixtures And Pure Substances Heterogeneous Mixture Pure Products Mixtures

How To Identify Heterogeneous Homogeneous Mixtures Food Snacks Good Carbs
0 Response to "are homogeneous mixtures always pure substances"
Post a Comment